There is often confusion as to how to choose the proper SPE ion exchange media. How do you determine if you should be looking at anion or cation, weak or strong? In this post, I will give some simple guidelines on making the best choice. You will see that ion exchange is all about acids and bases.
One thing that has often helped me remember is the saying “Strong is for weak and weak is for strong”. What this means is that strong ion exchange is for weak acidic or basic analytes while weak ion exchange is for strong acidic or basic analytes. This is true because if you have both a strong analyte and strong sorbent, you will usually have irreversible binding of your analyte and if you have a weak analyte with a weak sorbent, you usually won’t have enough charge to hold your analyte.
Definition of a strong and weak acid:
A strong acid will have a pKa < 1.0 while a strong base will have a pKa > 10. Strong acids and bases are 100% ionized while weak acids and bases are only partially ionized.
Sorbents:
Sulfonic acid modified sorbents used in strong cation exchange are strong acids while carboxylic acid modified sorbents used in weak cation exchange are weak acids. Cation exchange works best for basic analytes.
Quaternary modified amine sorbents used in strong anion exchange are strong bases while primary and secondary amine modified sorbents used in weak anion exchange are weak bases. Anion exchange works best for acidic analytes.

Table 1. Ion exchange media to use for different analyte types.
Because the sorbents in the strong ion exchange SPE columns have strong acid or base groups, these sorbents are 100% ionized at all pH’s. Therefore, to control the charge, the pH of the analyte needs to be adjusted to turn it on or off.
Because the sorbents in weak ion exchange SPE columns have weak acid or base groups, these sorbents are only partially ionized, depending on their pH. Because “strong” analytes are 100% charged at all pH’s, the pH of the sorbent needs to be adjusted to turn the charge on or off.
The control of charge when using strong ion exchange is performed by following the 2-pH unit rule of thumb. At the pKa of the analyte, it is 50% ionized. With acidic compounds, the analyte is 100% ionized at 2-pH units above the analytes pKa and fully neutral at 2-pH units below. For basic analytes, the process is reversed, the analytes are 100% ionized at 2-pH units below the pKa and 100% neutral at 2-pH units above the analytes pKa. The Acid/Base plot below shows an acid and base plot of an analyte with a pKa of 8.
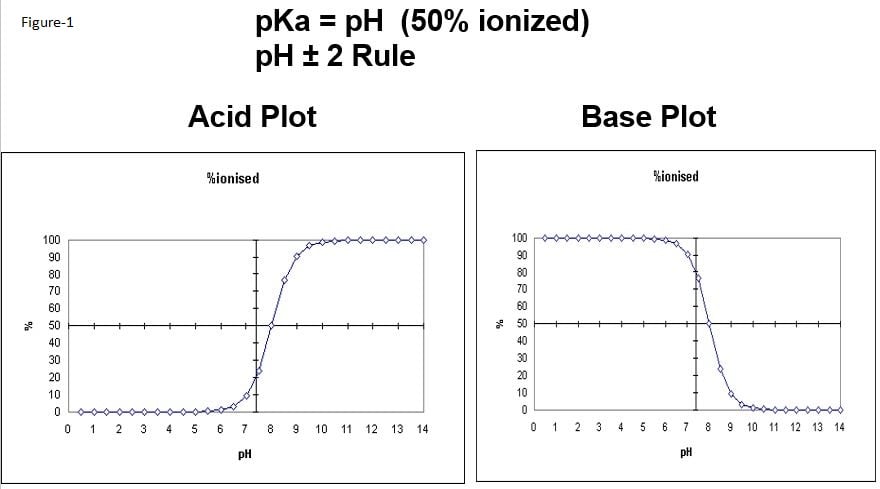
Figure 1. Acid and base plots of an analyte with pKa=8.
With strong ion exchange, you want to load with your analytes in a charged state so they can cling strongly to the sorbent during washing steps. This is carried out by properly pre-treating the sample to the proper pH and maintaining that pH during wash steps. During elution, you of course need to remove the charge on the analyte so it can come off for collection by changing the pH 2 units to the other side of the pKa. Again, this is done by adjusting the pH during the elution step using the 2-pH unit rule.
With weak ion exchange, you want to load with your sorbent in a charged state because the “strong” analyte is always 100% ionized, the pH of the sorbent is adjusted rather that the pH of the analyte to control charge. This is also performed by adjusting the pH during pre-treatmet and maintained during wash steps.
Because you are now turning on and off the charge of the sorbent rather than the analyte, the processing steps for strong anion exchange are similar to weak cation exchange and the steps to weak anion exchange are similar to strong cation exchange. You are still following the 2-pH unit rule of thumb only now as it relates to the sorbent rather than the analyte. Figure 2 below shows some examples of the treatment steps for the EVOLUTE® EXPRESS ion exchange SPE.
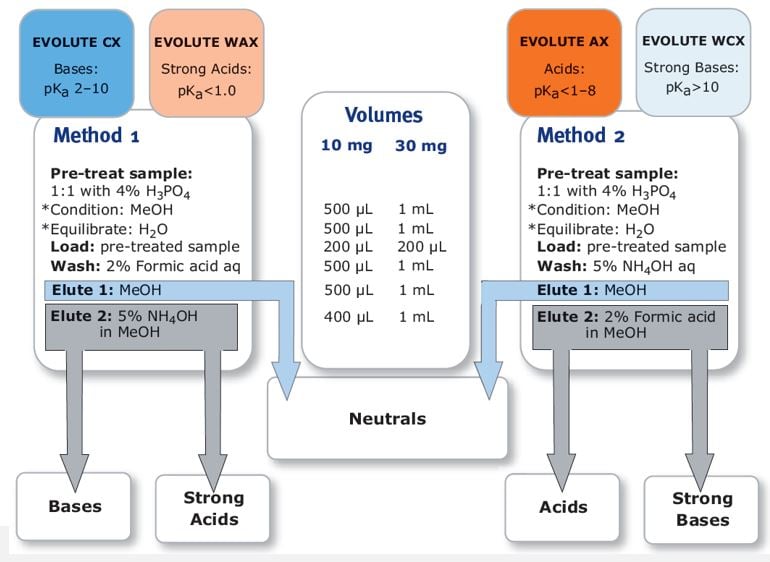
Figure 2. Examples of the treatment steps for the EVOLUTE® EXPRESS ion exchange SPE.
Do you want to know more about sample preparation? We have prepared for you plenty of interesting resources on extraction techniques and method optimization.
I hope this post has been helpful in your understanding of using ion exchange SPE. Do you want to know more about weak ion exchange SPE phases?

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership